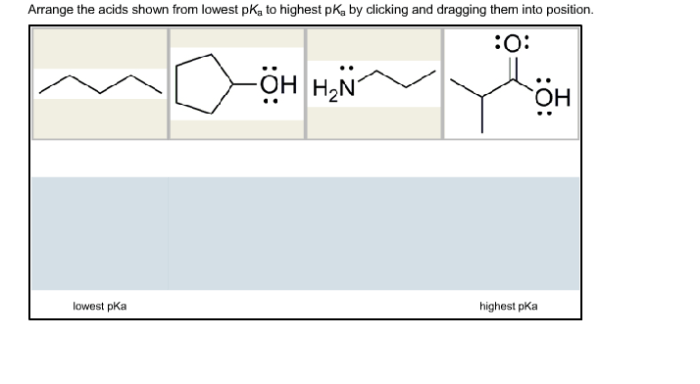
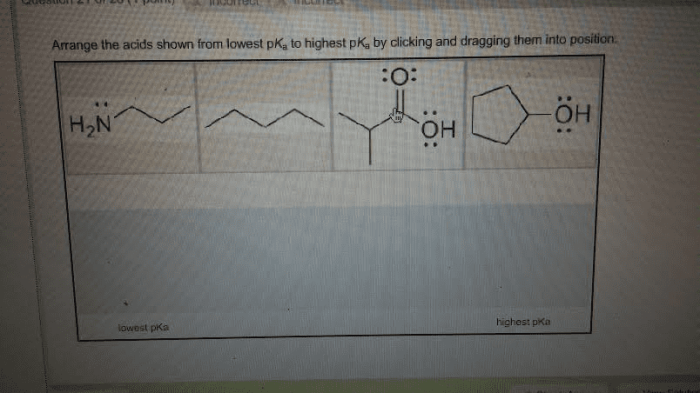
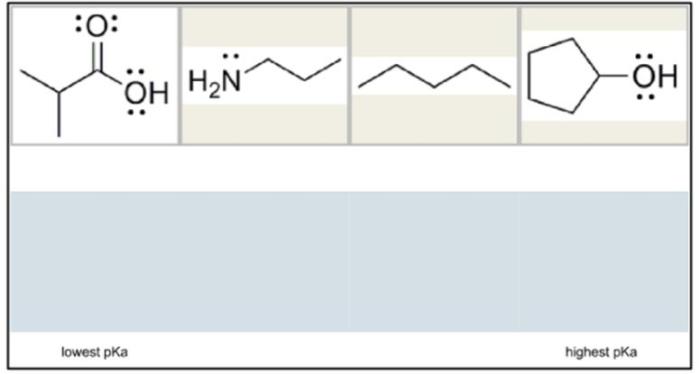
Arrange the acids shown from lowest pKa to highest pKa. Understanding the concept of pKa is crucial for comprehending acid strength and its significance in various chemical applications. This guide will delve into the intricacies of pKa, exploring its relationship with acid strength and providing a systematic approach to arranging acids based on their pKa values.
pKa, the negative logarithm of the acid dissociation constant, serves as a quantitative measure of acid strength. A lower pKa value indicates a stronger acid, as it readily releases protons. Conversely, a higher pKa value corresponds to a weaker acid with a lower tendency to dissociate.
Acid Dissociation Constant (pKa)
The acid dissociation constant (pKa) is a measure of the strength of an acid in a given solvent. It is defined as the negative logarithm of the equilibrium constant for the dissociation of an acid into its conjugate base and hydrogen ion (H+).
A lower pKa value indicates a stronger acid, as it means that the acid dissociates more readily in solution. Conversely, a higher pKa value indicates a weaker acid, as it dissociates less readily.
Arranging Acids by pKa
Acids can be arranged from lowest pKa to highest pKa, which corresponds to increasing acid strength. This arrangement is based on the equilibrium constant for the dissociation of the acid, with acids having a lower equilibrium constant (i.e., dissociating more readily) having a lower pKa.
By arranging acids in this manner, we can compare their relative strengths and predict their behavior in different chemical reactions.
Factors Influencing pKa
The pKa of an acid is influenced by several factors, including:
- Electronegativity of the atom bonded to the hydrogen: The more electronegative the atom bonded to the hydrogen, the stronger the acid. This is because the electronegative atom pulls electron density away from the hydrogen, making it more likely to dissociate.
- Resonance stabilization of the conjugate base: If the conjugate base of an acid can be stabilized by resonance, the acid will be stronger. This is because resonance delocalizes the negative charge of the conjugate base, making it more stable and less likely to recombine with the hydrogen ion.
- Solvent effects: The solvent can also affect the pKa of an acid. For example, acids are generally stronger in water than in nonpolar solvents.
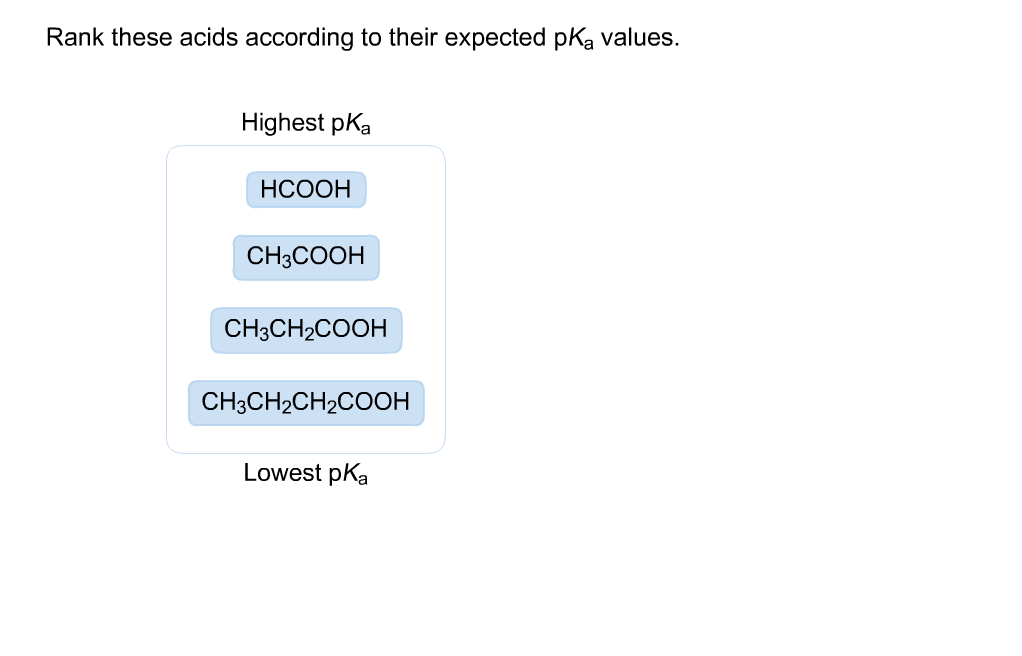
Examples of Acid Arrangement by pKa
| Acid | Formula | pKa |
|---|---|---|
| Hydrofluoric acid | HF | 3.17 |
| Acetic acid | CH3COOH | 4.76 |
| Hydrochloric acid | HCl | -7.0 |
| Sulfuric acid | H2SO4 | -3.0 |
Methods for Determining pKa, Arrange the acids shown from lowest pka to highest pka.
There are several experimental methods that can be used to determine the pKa of an acid, including:
- Potentiometric titration: This method involves titrating the acid with a strong base and measuring the pH of the solution at different points during the titration. The pKa can then be calculated from the pH at the equivalence point.
- Spectrophotometry: This method involves measuring the absorbance of a solution of the acid at different wavelengths. The pKa can then be calculated from the wavelength at which the absorbance is maximum.
- NMR spectroscopy: This method involves measuring the chemical shift of the hydrogen atoms in the acid. The pKa can then be calculated from the difference in chemical shift between the hydrogen atoms in the acid and in the conjugate base.
Applications of pKa in Chemistry
The pKa of an acid has many practical applications in chemistry, including:
- Buffer solutions: Buffer solutions are solutions that resist changes in pH. They are used in a wide variety of applications, including biological systems, analytical chemistry, and industrial processes.
- Acid-base reactions: The pKa of an acid can be used to predict the products and equilibrium constants of acid-base reactions.
- Solubility of ionic compounds: The pKa of an acid can be used to predict the solubility of ionic compounds in water.
- Drug design: The pKa of a drug can affect its absorption, distribution, metabolism, and excretion (ADME) properties. This information is important for designing drugs that are effective and safe.
Query Resolution: Arrange The Acids Shown From Lowest Pka To Highest Pka.
What is the significance of pKa in acid-base chemistry?
pKa plays a crucial role in determining the strength of an acid and predicting the extent of acid-base reactions. It provides a quantitative measure of the acidity of a solution and helps in understanding the behavior of acids in various chemical processes.
How can I experimentally determine the pKa of an acid?
Several experimental methods are available for determining the pKa of acids, including potentiometric titration, spectrophotometry, and conductometry. Each method utilizes different principles and procedures to measure the dissociation constant of the acid.
What factors influence the pKa of an acid?
The pKa of an acid is influenced by various factors, such as the electronegativity of the atoms involved, the hybridization of the atoms, the resonance effects, and the solvent effects. These factors collectively determine the stability of the conjugate base and, consequently, the pKa of the acid.